Pages
Health Care News
Categories
- Asthma education
- Autism
- Canadian Health&Care Mall
- Cardiac function
- Critical Care Units
- Follicle
- Health
- health care medical transport
- health care programs
- Health&Care Professionals
- Hemoptysis
- Hormone
- Isoforms
- Nitroglycerin Patches
- Profile of interleukin-10
- Progesterone
- Pulmonary Function
- Sertoli Cells
- Theophylline
- Tracheoesophageal Fistula
Category Archives: Follicle
Mammalian Oogenesis and Folliculogenesis: DISCUSSION(9)
 However, in contrast to the situation described for rats, the spontaneous activation of rabbit primordial follicles was not inhibited by KITL-neutralizing antibody, suggesting that endogenous KITL does not contribute to the spontaneous activation of primordial follicles in rabbit ovarian cortical explants in vitro. Caution must be applied in the interpretation of these results, as it is possible that this antibody does not inhibit KIT signal transduction in rabbits, as is suggested by our results showing increased rabbit oocyte growth in the presence of combined recombinant KITL protein and neutralizing KITL antibody.
(more…)
However, in contrast to the situation described for rats, the spontaneous activation of rabbit primordial follicles was not inhibited by KITL-neutralizing antibody, suggesting that endogenous KITL does not contribute to the spontaneous activation of primordial follicles in rabbit ovarian cortical explants in vitro. Caution must be applied in the interpretation of these results, as it is possible that this antibody does not inhibit KIT signal transduction in rabbits, as is suggested by our results showing increased rabbit oocyte growth in the presence of combined recombinant KITL protein and neutralizing KITL antibody.
(more…) Mammalian Oogenesis and Folliculogenesis: DISCUSSION(8)
Alternatively, it has been proposed that the ovarian medullar may produce a factor that retards follicle activation.
Further insight into the phenomenon of spontaneous follicle activation has been generated from studies demonstrating that primordial follicles in bovine and baboon cortical explants grafted to chick embryonic membranes are not spontaneously recruited.
(more…)
Mammalian Oogenesis and Folliculogenesis: DISCUSSION(7)
 The results of the current study, however, were unable to support these latter findings.
The spontaneous activation of primordial follicles in cultured ovarian tissue has been observed for a number of species, including cattle, primates, and, to a much lesser extent, rats. It is unknown why primordial follicles are triggered to spontaneously activate when maintained in vitro, although clues as to the mechanism are beginning to emerge. In vivo, primordial follicles exit the resting pool in a gradual manner.
(more…)
The results of the current study, however, were unable to support these latter findings.
The spontaneous activation of primordial follicles in cultured ovarian tissue has been observed for a number of species, including cattle, primates, and, to a much lesser extent, rats. It is unknown why primordial follicles are triggered to spontaneously activate when maintained in vitro, although clues as to the mechanism are beginning to emerge. In vivo, primordial follicles exit the resting pool in a gradual manner.
(more…) Mammalian Oogenesis and Folliculogenesis: DISCUSSION(6)
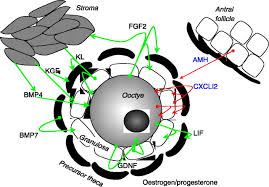
Therefore, an upstream signal may be required to induce the granulosa cells to generate threshold KITL levels before follicle activation can proceed. This signal may be a reduction in inhibitors, an increase in activators, or a combination of both.
KITL has been reported to be a survival factor for a number of different cell types, including hematopoietic stem cells, spermatogonia, and primordial germ cells.
(more…)
Mammalian Oogenesis and Folliculogenesis: DISCUSSION(5)
 Consistent with this view is the observation that a number of mutations at both the white spotting and steel loci have severe reproductive phenotypes in mice, whereas even though a number of human diseases have been associated with defects in these genes, none have been described with reproductive phenotypes. Furthermore, Fortune et al. in a review quote data to indicate that KITL does not promote follicle activation in bovine ovarian cortical explants.
(more…)
Consistent with this view is the observation that a number of mutations at both the white spotting and steel loci have severe reproductive phenotypes in mice, whereas even though a number of human diseases have been associated with defects in these genes, none have been described with reproductive phenotypes. Furthermore, Fortune et al. in a review quote data to indicate that KITL does not promote follicle activation in bovine ovarian cortical explants.
(more…) Mammalian Oogenesis and Folliculogenesis: DISCUSSION(4)
Therefore, the primary role of KITL in this species may be to promote oocyte growth in follicles that have already received the signal(s) to activate.
Similar to rabbits, exogenous KITL stimulated the growth of oocytes in cultured neonatal mouse ovaries, but the growth-promoting effects were more stage restricted, with only primordial and early primary follicles affected.
(more…)
Mammalian Oogenesis and Folliculogenesis: DISCUSSION(3)
 We, therefore, employed an in vitro approach to investigate the effect of KITL on follicle survival, oocyte growth, and follicle recruitment in rabbit ovarian cortical explants and neonatal mouse ovaries, and we found striking differences between the rabbit and mouse model systems regarding the effect of exogenous KITL supplementation on these parameters. In rabbit ovarian cortical explants treated with recombinant mouse KITL there was a significant and dose-dependent increase in oocyte diameter.
(more…)
We, therefore, employed an in vitro approach to investigate the effect of KITL on follicle survival, oocyte growth, and follicle recruitment in rabbit ovarian cortical explants and neonatal mouse ovaries, and we found striking differences between the rabbit and mouse model systems regarding the effect of exogenous KITL supplementation on these parameters. In rabbit ovarian cortical explants treated with recombinant mouse KITL there was a significant and dose-dependent increase in oocyte diameter.
(more…) Mammalian Oogenesis and Folliculogenesis: DISCUSSION(2)
In the rabbit, Kitl mRNA and protein were detected in the squamous granulosa cells of some primordial follicles. The cuboidal granulosa cells surrounding growing follicles uniformly expressed high levels of KITL until the antral stage of development, when the presence of KITL became sporadic. Both mRNA and protein for KIT were detected within all oocytes, at every stage of development. In general, the amount of KIT protein localized to the oolemma increased with follicle growth and then decreased within the fully grown oocytes of antral follicles.
(more…)
Mammalian Oogenesis and Folliculogenesis: DISCUSSION(1)
 Primordial follicles are the stores of female gametes from which all mature eggs for ovulation and fertilization arise. The recruitment of primordial follicles into the growth phase is tightly controlled and likely involves the action of both stimulatory and inhibitory molecules. Although the exact mechanisms that regulate primordial follicle activation and the initiation of oocyte growth remain elusive, the study of rodent models has shown KITL and KIT to be involved in these processes.
(more…)
Primordial follicles are the stores of female gametes from which all mature eggs for ovulation and fertilization arise. The recruitment of primordial follicles into the growth phase is tightly controlled and likely involves the action of both stimulatory and inhibitory molecules. Although the exact mechanisms that regulate primordial follicle activation and the initiation of oocyte growth remain elusive, the study of rodent models has shown KITL and KIT to be involved in these processes.
(more…) Mammalian Oogenesis and Folliculogenesis: RESULTS(19)
In cultured untreated ovaries, oocytes from primordial follicles were considerably larger (P < 0.05) than those of primordial follicles in preculture tissues, whereas the mean diameters of oocytes from follicles at all other stages of development were unaffected by culture (Table 2). The spontaneous enlargement of oocytes from primordial follicles was not antagonized by KITL-neutralizing antibody (Table 2), suggesting that some unknown aspect of culture other than endogenous KITL induced the observed growth.
(more…)
