Pages
Health Care News
Categories
- Asthma education
- Autism
- Canadian Health&Care Mall
- Cardiac function
- Critical Care Units
- Follicle
- Health
- health care medical transport
- health care programs
- Health&Care Professionals
- Hemoptysis
- Hormone
- Isoforms
- Nitroglycerin Patches
- Profile of interleukin-10
- Progesterone
- Pulmonary Function
- Sertoli Cells
- Theophylline
- Tracheoesophageal Fistula
Category Archives: Follicle
Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(9)
 In every third section follicles were counted and scored for stage of development, and the diameter of each oocyte was measured across the largest cross-section. Of follicles deemed healthy, only those with a visible germinal vesicle were counted or measured. The mean diameter of germinal vesicles for the mouse ranged from 9.6 6 0.01 im to 19.8 6 2.6 im (primordial to preantral follicles), and for the rabbit from 19.2 6 0.01 im to 22.2 6 0.03 im (primordial to primary follicles).
(more…)
In every third section follicles were counted and scored for stage of development, and the diameter of each oocyte was measured across the largest cross-section. Of follicles deemed healthy, only those with a visible germinal vesicle were counted or measured. The mean diameter of germinal vesicles for the mouse ranged from 9.6 6 0.01 im to 19.8 6 2.6 im (primordial to preantral follicles), and for the rabbit from 19.2 6 0.01 im to 22.2 6 0.03 im (primordial to primary follicles).
(more…) Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(8)
Both the recombinant mouse KITL protein and neutralizing antibody were obtained from Sigma Chemical Co. Doses of recombinant mouse KITL were selected on the basis of concentrations previously shown to promote oocyte growth and follicle activation in the mouse and rat, respectively. The dose of neutralizing antibody was determined using the ED50 provided by the manufacturer. In similar experiments rabbit ovarian explants were incubated with 150 ng/ml of recombinant rabbit KITL (see Supplemental Table 2).
(more…)
Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(7)
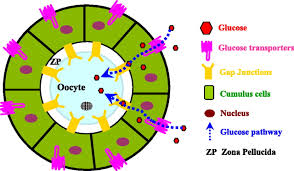 Ovaries and explants were cultured on collagen I- and fibronectin-coated nitrocellulose membranes (0.45-im pore size; Millipore Corp., Bedford, MA) and mounted on triangular metal grid supports in Falcon Organ Culture Dishes (Becton Dickinson Co., Franklin Lakes, NJ). Coating was performed by incubating the membranes in 0.75 mg/ml collagen and 0.1 mg/ml fibronectin for 1 h at 37°C. Ovaries and ovarian explants were cultured in groups of three to five for 8 days in Dulbecco Modified Eagle medium (DMEM)/Ham F12 1:1 (v/v) (GibcoBRL, MD) supplemented with 10% fetal bovine serum, 0.03 IU recombinant FSH (Gonal-F 75; Serono, Italy), 5 ig/ml insulin, 5 ig/ml transferrin, 5 ng/ml selenium, and 0.1 iM retinoic acid.
(more…)
Ovaries and explants were cultured on collagen I- and fibronectin-coated nitrocellulose membranes (0.45-im pore size; Millipore Corp., Bedford, MA) and mounted on triangular metal grid supports in Falcon Organ Culture Dishes (Becton Dickinson Co., Franklin Lakes, NJ). Coating was performed by incubating the membranes in 0.75 mg/ml collagen and 0.1 mg/ml fibronectin for 1 h at 37°C. Ovaries and ovarian explants were cultured in groups of three to five for 8 days in Dulbecco Modified Eagle medium (DMEM)/Ham F12 1:1 (v/v) (GibcoBRL, MD) supplemented with 10% fetal bovine serum, 0.03 IU recombinant FSH (Gonal-F 75; Serono, Italy), 5 ig/ml insulin, 5 ig/ml transferrin, 5 ng/ml selenium, and 0.1 iM retinoic acid.
(more…) Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(6)
The tissue sections (four from each ovary) were deparaffinized in xylene and then rehydrated in alcohol. Pretreatment, hybridization, and posthybridization washes were performed according to protocols described in the Non-radioactive In Situ Hybridization Application Manual (Roche Diagnostics). Kitl sense and antisense probes were hybridized overnight at 50°C. Kit sense and antisense probes were hybridized overnight at 68°C. Probes were detected immunohistochemically using the DIG Nucleic Acid Detection Kit (Roche Diagnostics) according to protocols described by the manufacturer.
(more…)
Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(5)
 The tissues were then incubated with biotin-conjugated goat anti-rat immunoglobulin G (IgG; Sigma Chemical Co.) diluted 1:1000 in PBS for 1 h at 37°C. Antibody binding was visualized using the ImmunoPure ABC Peroxidase Staining Kit (Pierce, Rockford, IL) and the Liquid DAB-Plus Substrate Kit (Zymed, San Francisco, CA). Sections were counterstained with hematoxylin and visualized by light microscopy using an Olympus BH-2 microscope. Negative controls, in which the primary antibody was replaced with preimmune serum, were routinely performed on adjacent serial sections, and no specific staining was observed.
(more…)
The tissues were then incubated with biotin-conjugated goat anti-rat immunoglobulin G (IgG; Sigma Chemical Co.) diluted 1:1000 in PBS for 1 h at 37°C. Antibody binding was visualized using the ImmunoPure ABC Peroxidase Staining Kit (Pierce, Rockford, IL) and the Liquid DAB-Plus Substrate Kit (Zymed, San Francisco, CA). Sections were counterstained with hematoxylin and visualized by light microscopy using an Olympus BH-2 microscope. Negative controls, in which the primary antibody was replaced with preimmune serum, were routinely performed on adjacent serial sections, and no specific staining was observed.
(more…) Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(4)
Two booster injections of protein (50 ig), dissolved in PBS (50 il) and emulsified in an equal volume of Freunds Incomplete Adjuvant, were given at 2-wk intervals, commencing 4 wk after priming injections. Fourteen days following the final boost, rats were anesthetized with carbon dioxide and then exsanguinated by cardiac puncture. Blood sera were collected and tested for specificity against the respective immunogens and commercially obtained protein (recombinant mouse SCF; Sigma Chemical Co.) by Western blot (not shown).
(more…)
Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(3)
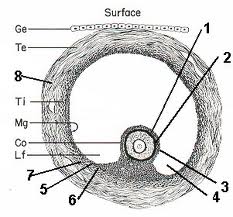 PCR primers, plasmids, and bacteria used for cloning and recombinant protein expression are described (see Supplemental Table 2, available online at: http:// www.biolreprod.org/). Recombinant proteins were affinity purified with Ni-NTA resin (Qiagen, Australia) and renatured by dialysis using protocols described in The QIAexpressionist (Fifth edition, Qiagen; http://www1.qiagen. com/literature/handbooks/PDF/Protein/Expression/QXP_QIAexpressionist/ 1024473_QXPHB_0603.pdf).
(more…)
PCR primers, plasmids, and bacteria used for cloning and recombinant protein expression are described (see Supplemental Table 2, available online at: http:// www.biolreprod.org/). Recombinant proteins were affinity purified with Ni-NTA resin (Qiagen, Australia) and renatured by dialysis using protocols described in The QIAexpressionist (Fifth edition, Qiagen; http://www1.qiagen. com/literature/handbooks/PDF/Protein/Expression/QXP_QIAexpressionist/ 1024473_QXPHB_0603.pdf).
(more…) Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(2)
Conditions for PCR were as follows: 1X (3 min at 94°C); 30X (94°C for 45 sec, 55°C for 1 min, 72°C for 1 min); 1X (72°C for 5 min). PCR products were ligated into the pGEM-T Easy vector (Promega Corp., Australia) and cloned into XL-1 Blue Escherichia coli (Stratagene, La Jolla, CA). Cloned cDNAs were sequenced (Biomolecular Resource Facility, JCSMR, ANU), and nucleotide and amino acid sequence analyses were performed using Lasergene v6 software (DNASTAR Inc., Madison, WI). Sequences were deposited in GenBank, and accession numbers for rabbit Kitl DQ356265, rabbit Kit (cytoplasmic domain) DQ356267, and rabbit Kit (extracellular domain) DQ356266 were assigned respectively.
(more…)
Mammalian Oogenesis and Folliculogenesis: MATERIALS AND METHODS(1)
 Materials
Unless otherwise stated, reagents were obtained from Sigma Chemical Co. (Australia).
Animals
All animal experimental procedures were approved by the CSIRO, Division of Sustainable Ecosystems, Gungahlin, Animal Experimentation Ethics Committee in accordance with National Health and Medical Research Council/CSIRO guidelines.
(more…)
Materials
Unless otherwise stated, reagents were obtained from Sigma Chemical Co. (Australia).
Animals
All animal experimental procedures were approved by the CSIRO, Division of Sustainable Ecosystems, Gungahlin, Animal Experimentation Ethics Committee in accordance with National Health and Medical Research Council/CSIRO guidelines.
(more…) Mammalian Oogenesis and Folliculogenesis: INTRODUCTION(4)
Given the limited accessibility of human ovarian tissue and the lack of information available for the activity of KITL in species other than the mouse, we identified the rabbit as an alternative animal model for the study of the roles of KITL and KIT during the early stages of folliculogenesis. The initial objective of this research was to use in situ hybridization and immunohistochemistry to provide a detailed analysis of Kitl and Kit mRNA and protein expression within the ovaries of rabbits during the assembly of primordial follicles and the first wave of folliculogenesis, which occurs between Weeks 2 and 12 of postnatal development in this species.
(more…)
